Throughout the many shut-downs of the pandemic, we have continued to work toward treatments for CMT4J. Our researchers and scientists have shown incredible grit in persevering through immeasurable delays and roadblocks. And while thousands of dedicated folks worked on treatments and vaccines for Covid, our scientific team rolled up their sleeves and continued working on another little known disease that also has devastating effects for patients and families.
I’m pleased to share our research update, providing some insight into the many different treatment pathways we’re pursuing and how hard we’re all working to get to a treatment for CMT4J.
CMT4J RESEARCH PATHWAYS and PARTNERS:
1. FIG4/AAV9 GENE THERAPY– Neurogene
Although Covid has delayed most rare disease clinical trials and drug manufacturing, we are encouraged to know that Neurogene continues to push forward toward a clinical trial. Our CMT4J Natural History Study, taking place at the University of Texas-Southwestern and the University of Iowa, allows patients to be seen either in person or virtually. The natural history study provides an essential opportunity to better understand such a rare disease and, ultimately, a way to show that potential treatments work and make a difference in the lives of CMT4J patients.

WHY IS IT TAKING SO LONG? Initially, Neurogene encountered manufacturing problems in the original construct of the gene therapy medicine—it made enough for tiny mice, but couldn’t produce enough for humans. As soon as Neurogene solved that problem, Covid hit, causing delays in every possible aspect. Borders and shipping shut down for most biologics. Materials needed to manufacture gene therapies were nowhere to be found; even plastic for research materials such as pipettes are STILL in short supply. And nearly every lab that was not working on Covid was ordered to cease operation. In addition, the FDA has put out more stringent guidelines for gene therapy trials, in an effort to ensure safety. This makes the approval process for clinical trials even more complicated and delayed. Finally, the manufacturing process for gene therapies has had a tremendous backlog for years now, long before Covid hit. There simply aren’t enough manufacturing facilities to handle the demand. In response to this, Neurogene is developing their own manufacturing facility. Ultimately, this will allow them to manufacture gene therapies faster and more cheaply, but unfortunately, takes time to build. Neurogene recently shared with our CMT4J community that they look to 2022 to begin manufacturing gene therapies.
2. UNIVERSITY OF MICHIGAN MEISLER LAB:

Because of your support, CureCMT4J provided a sponsored research grant to the Meisler Lab —home to the two leading FIG4 gene experts in the world! This grant has enabled us to fund multiple, simultaneous treatment development pathways. CureCMT4J also funded the hire of Dr. Xu Cao, paving the way for another FIG4 expert!
Meisler Lab Fast-Track Treatment Pathway Research:
A. Affiliate-Gene Networking – the FIG4 gene interacts with several other different genes to make the protein necessary for sustaining healthy nerves and cellular-related pathways. The Meisler Lab has discovered that if one of these genes is inhibited it may actually help compensate for the loss of FIG4, giving back some of the missing function, resulting in healthier cells. UMich is using this treatment approach when looking at both existing drugs that have an effect on these “neighboring” genes, as well as gene editing techniques to either upregulate or down-regulate them.
B. Drug Repurposing and “Low Throughput Screens” – we’re working day and night to identify existing drugs (already FDA-approved for other diseases) that also might help to treat or halt the symptoms of CMT4J. Our UMich researchers are currently working on cell or mouse experiments on at least five different drugs.
C.ASO (antisense oligonucleotide) Development – it is well known that a healthy FIG4 gene generates something called phosphoinositide, or PI(3,5)P2, which regulates lysosomal function within cells. When the gene is defective—through genetic mutations or variants—these lysosomes and the cells in which they reside, stop working and build up with garbage. This causes nerve cells to die off, resulting in neuromuscular weakness that we see in CMT4J. Recently, UMich researchers identified yet another gene involved with this process. Dr. Guy Lenk, Dr. Miriam Meisler, and Dr. Xu Cao hope to prove out these gene relationships, with the aim of developing an ASO—a synthetic RNA “bandaid” that might help to increase the missing FIG4 protein.
3. DRUG REPURPOSING USING HIGH THROUGHPUT SCREEN- MODELIS
We are thrilled to announce our latest research project and partnership! Modelis uses High Throughput Screening (HTS) in worm and fish models. Using a library of 4,000+ FDA-approved drugs and other stalled or shelved phase 3/phase 4 drugs, Modelis screens them in “CMT4J worm” or fish models to look for a drug that might halt and/or treat the disease. Identifying an existing drug or drug-drug combination drastically cuts time and cost in getting into humans. Thank you for supporting the funding for this cutting edge treatment development project!

From Modelis CEO, James Doyle:
“At Modelis we value the disease-specific expertise from rare disease parents and patients in order to help guide & shape our program. We can’t identify or develop treatments without them!”
4. THE JACKSON LABORATORY
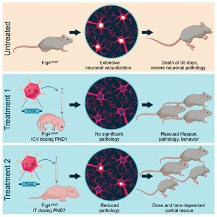
Did you happen to see our recent posts, sharing the news that our gene therapy preclinical work at JAX was finally published? Dr. Cat Lutz, Dr. Robert Burgess, and Dr. Maximillion Presa, along with Dr. Steven Gray, Dr. Rachel Bailey, Dr. Jun Li, and Dr. Guy Lenk worked tirelessly to address every last question related to safety and efficacy. The published work is an enormous milestone in the greater Charcot Marie Tooth (CMT) research community. Our work has been presented at multiple scientific and medical conferences as a model approach to tackling gene therapies for rare diseases. We are so proud to have spear-headed and co-funded the work, along with a prestigious grant to JAX from the NIH (National Institutes of Health). You can find the paper in The Journal of Clinical Investigation.
Additionally, we continue to work with our CMT4J researchers at JAX, this time, looking at another gene recently identified in numerous papers as having an effect on neurodegenerative processes. We’re testing the hypothesis on our CMT4J mouse models at JAX and hope to show that this is yet another (easier) pathway to pursue treatments for CMT4J.
5. THE BROAD INSTITUTE

In 2020, our amazing researcher at The Broad—Samantha Baxter—worked with us on an Aggregate Frequency Analysis project for CMT4J. Translation: just how many CMT4J patients should we expect to be out there in the world? This research was awarded to us through an ultra-rare disease grant for genes of interest. After many months and a lengthy process of calculations, data collection and algorithm analysis, we learned that our CMT4J patient community is actually much, much larger than we had initially thought. Currently, we’ve identified approximately 50-60 patients worldwide. Imagine our surprise when those calculations revealed that 6,000-9,000 CMT4J patients should actually exist! Most, or many, are as yet undiagnosed, or misdiagnosed, as has been the case for so many people with CMT4J. These numbers, of course, still make CMT4J a super-rare disease, but if we can find more patients, we could possibly help and save more lives with future treatments, and our ability to partner with companies on treatment development and clinical trials is made much easier.
6. DRUG REPURPOSING II

Through our own research CureCMT4J discovered the link between one of the FIG4 “partner” genes and its effect on neurological and neurodegenerative diseases. An existing vitamin supplement supports and enhances the cellular pathways involved with these genes. Our suspicions have been confirmed through research in multiple, related diseases, as well as in CMT4J cells! We have since shared this news with our patient community and are cautiously optimistic that taking the supplement may help CMT4J patients.
THANK YOU!
Every single day, we continue to research, identify, and push forward potential treatments for individuals with CMT4J. We’ve learned over the past few years that, unfortunately, it isn’t a sprint to the finish line. It is a marathon. It is a slog. Science is rarely straightforward. Regulatory roadblocks grow larger every year. Research funding and manufacturing delays still represent the biggest hurdles for ultra-rare diseases. We are one of the lucky ones, to have a biopharma further our gene therapy trial—current estimates run near $6-10MM to do what we have done and get through a phase I/II trial. It is not without sacrifice, for sure, as we lose control of timelines and decision-making. And then, a global pandemic brings us to our knees.
To watch the horrific progression of this disease in my own child, with the backdrop of a potential treatment just out of reach is devastating, maddening and heartbreaking. Words have failed me over the past many months. Some days it is difficult to find a way forward. But that, essentially, is at the heart of our mission—finding the way forward.
HOW CAN YOU HELP?
Does your child or do you have CMT4J and aren’t yet enrolled in the CMT4J Natural History Study?
Please consider taking part, either remotely or in person. You can find information about the natural history study here, at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT03810508?cond=CMT4J&rank=1
Or reach out to Neurogene here via email or phone: medicalinfo@neurogene.com // 877-237-5020
We need you!
We always need help! If you believe you have expertise and interest in any of the following areas, we’d love to hear from you:
Research/Science – we could always use some extra eyes to scan and scour the medical literature, looking for relatable research papers and approaches to treatments being used by similar diseases. Do you have experience in medicine, research, bioinformatics, or epidemiology? Can you read and interpret scientific studies? Please reach out at jocelynaduff@gmail.com
Web Conferencing/Tech Support – We’re working on a virtual CMT4J patient and family conference. Our hope is to connect patients and families, and include a panel of experts/guest speakers to provide updates on research and other topics. Do you have experience in designing and running/hosting web conferences? Reach out to us here: jocelynaduff@gmail.com
Fundraising– Want to host a fundraiser for CureCMT4J? Go to our website for some ideas: http://www.curecmt4j.org/get-involved/.
SHOELACES!
We have lots of CureCMT4J shoelaces if you’d like to use them in your fundraiser! (suggested donation $5/pair [individual non-event lace orders = $5/pair + shipping) Contact us at: jocelynaduff@gmail.com
FACEBOOK BIRTHDAY FUNDRAISERS!
Want a simple way to fundraise for and spread the word about CureCMT4J ? If you have a Facebook account you can host a Birthday Fundraiser every year on your special day! Go here to find out how to do it: https://www.facebook.com/help/1910205189301966/ [Facebook receives no money raised in these online FB fundraisers]
Or DONATE on a one-time or monthly basis on our website!
Thank you!!!

Recent Comments