AAV9/FIG4 GENE THERAPY
CURECMT4J’s GENE THERAPY PROGRAM HAS BEEN SELECTED BY THE NATIONAL INSTITUTES OF HEALTH (NIH) BESPOKE GENE THERAPY (BGTC) PROGRAM!
The Accelerating Medicines Partnership® (AMP®) Program Bespoke Gene Therapy Consortium (AMP BGTC) brings together partners from the public, private, and non-profit sectors to foster development of gene therapies intended to treat rare genetic diseases, which affect populations too small for viable commercial development. The BGTC aims to overcome major obstacles related to developing gene therapies.
Want to know more? Visit the BGTC website here!
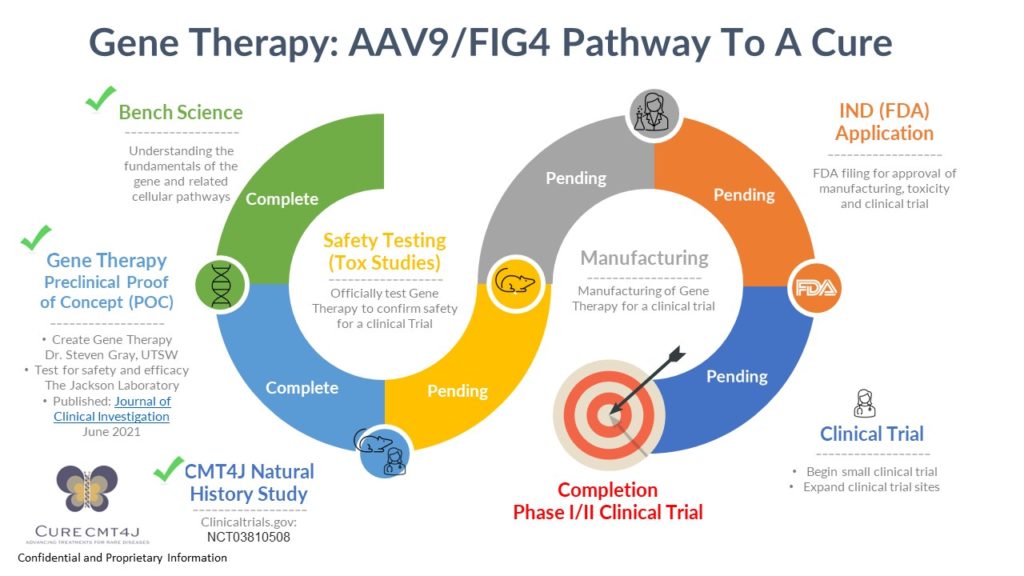
PRE CLINICAL WORK COMPLETE AND SUCCESSFUL!
Pre clinical work was extremely promising, showing that gene therapy halts progression and causes some reversal of symptoms of disease in young mice. CMT4J mouse models typically only live for 3-4 weeks. These same mouse models, when treated with AAV9/FIG4 gene therapy, lived a near-normal lifespan, showing none of the typical neurological signs associated with this CMT4J animal model. This work was funded by CureCMT4J and through a Precision Genetics Grant from the National Institutes of Health to The Jackson Laboratory. These results have been published in the esteemed Journal of Clinical Investigation. This preclinical work serves as the foundation for moving forward with a first-in-human clinical trial.
WHAT IS GENE THERAPY?
 Gene therapy is a way of treating genetic-based diseases by delivering healthy copies of the faulty gene. Scientists create a “viral vector”, which packages a healthy copy of the gene within a benign virus that will deliver the gene to appropriate cells throughout the body. Trillions of copies of the viral vector are made, then injected into the person, where they are then incorporated into cells. Researchers are hopeful that gene therapy for CMT4J has the potential to not only halt disease progression, but possibly reverse some of the symptoms and damage that has already occurred. Gene therapy is currently being tested in over 600 clinical trials.
Gene therapy is a way of treating genetic-based diseases by delivering healthy copies of the faulty gene. Scientists create a “viral vector”, which packages a healthy copy of the gene within a benign virus that will deliver the gene to appropriate cells throughout the body. Trillions of copies of the viral vector are made, then injected into the person, where they are then incorporated into cells. Researchers are hopeful that gene therapy for CMT4J has the potential to not only halt disease progression, but possibly reverse some of the symptoms and damage that has already occurred. Gene therapy is currently being tested in over 600 clinical trials.
The FDA approved gene therapy for a form of blindness in 2017 and in Spinal muscular atrophy (SMA) in 2019. On-going trials using gene therapy in other rare diseases have shown very promising results as well
How Do We Get There?
DRUG REPURPOSING
If we can identify an already-existing drug as a treatment, with a known safety profile, then getting that treatment into humans is so much easier and faster.
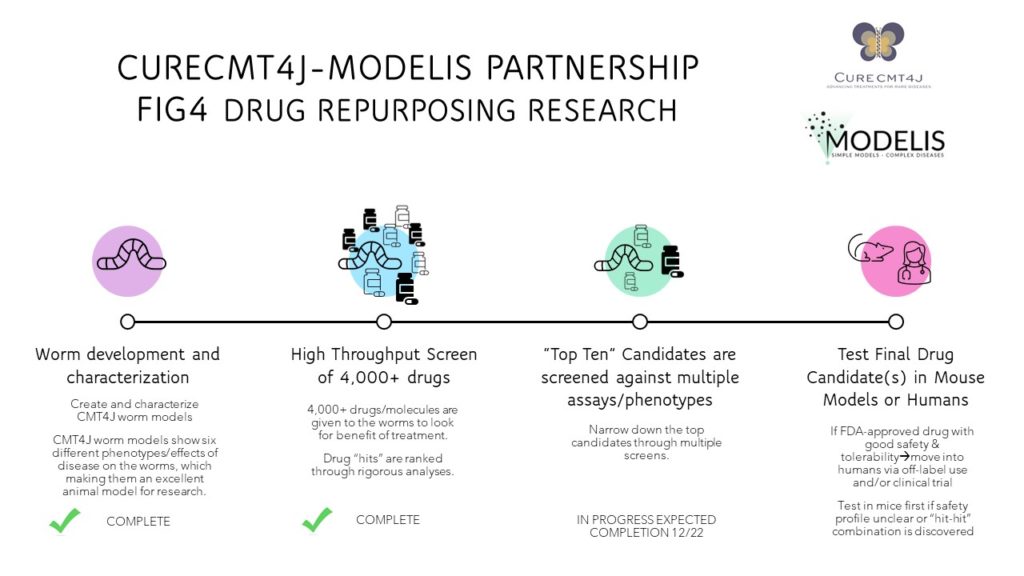
HIGH THROUGHPUT SCREENING (HTS) – HTS is a rapid way of testing thousands of drugs on disease models. CureCMT4J has funded and partnered with Modelis to make the first CMT4J/FIG4 worm model. We are in the process of narrowing down drug target “hits” after testing more than 4,000 drugs in the models. A treatment for CMT4J may already exist and could easily be repurposed to treat it. This is a much easier pathway for getting treatments to patients.
OTHER THERAPEUTIC PATHWAYS
UNIVERSITY OF MICHIGAN MEISLER LAB
CureCMT4J has funded research at the Meisler Lab to identify drugs to repurpose for CMT4J. Dr. Guy Lenk, Dr. Xu Cao, Dr. Miriam Meisler, and other researchers are also looking into affiliated gene pathways that might help to increase or stabilize the FIG4 gene.

Potential Treatment Development Areas:
ASO Feasibility study
Affiliated Gene Pathways
Upregulation of FIG4 pathways
Lysosomal storage/cellular mechanism corrections

____________________________________________________________________________________________________________________
CMT4J BIOMARKER INVESTIGATORY RESEARCH
CureCMT4J is working with The Jackson Laboratory, in their Rare and Orphan Disease Center–as well as with other entities and researchers–to identify potential biomarkers for CMT4J. Biomarkers are crucial in clinical trials in order to show that a drug is working.


Recent Comments